How to choose a good medical power supply?
2024/6/24 9:46:51Power supply is the heart of medical equipment energy. Its basic performance and potential application risks have a vital impact on the entire medical equipment. The first question for medical equipment manufacturers is, should they make their own or outsourced power supply? Frankly speaking, it is not difficult to design a power supply, but it is not easy to design and produce a high-quality medical power supply. Therefore, it is more cost-effective for medical equipment manufacturers to directly purchase a good medical power supply and free up their energy to focus on their own profession. So, what factors need to be considered in order to choose a good medical power supply?
1. Security and Isolation
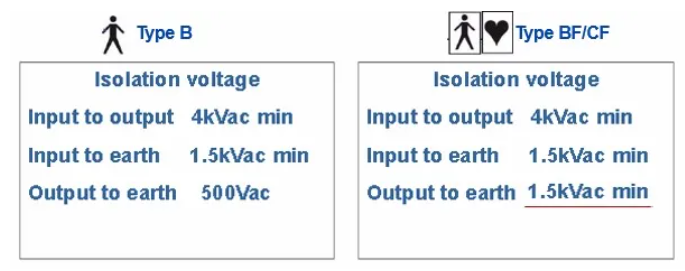
Currently, the medical standard implemented in Europe and the United States is UL/EN60601 version 3, while China implements GB9706.1-2007 version (equivalent to 60601 version 2) and is preparing to transition to the third edition.Medical gradepower supplies not only need to meet requirements for protection against electric shock and fire, but also need to be designed to meet higher electrical clearance, creepage distance and isolation voltage level requirements to ensure that medical personnel or patients are protected from any other risks such as electric shock. It is usually required that the input and output of the power supply meet 2xMOPPs. The isolation voltage requirements for different levels of medical devices are as follows:
2.Leakage current
The current that the human body can withstand is very weak, and tens of microamps can cause fatal damage to the heart. Therefore, the UL/EN60601 standard has strict requirements for the ground leakage current, contact current (the second edition is called shell leakage current), patient leakage current, patient auxiliary current and leakage current under various single fault conditions of medical equipment. So, how much leakage current power supply should we choose? Considering that under a single fault condition, the leakage current to the ground will become a contact current, the leakage current to the ground of the entire equipment must be less than 300uA. If the overall device contains filters, multiple power supplies, or there are input Y capacitors on the system, the leakage current generated will have a superimposed effect, so the power supply needs to be selected systematically. The leakage current limits for medical equipment (not permanently installed) are as follows:
3.EMC
Obviously, a medical power supply with sufficient EMC margin can lay a good foundation for the EMC of the entire machine, which will have the effect of getting twice the result with half the effort. At present, most medical equipment EMI needs to meet EN55011 Class B, or equivalent; for EMS, such as ESD, EFT, Surge, Dips, etc., Europe and the United States implement the fourth edition requirements, that is, IEC60601-1-2:2015, to ensure The basic safety and essential performance of medical devices and systems must not be interfered with by other devices. It is worth emphasizing that you must understand which level and criteria the power supply meets, because this is an important reference for risk assessment. Regarding the levels and judgment basis of medical power EMS testing, please refer to the following figure:

4.Lifespan
The design and certification cycle of medical equipment is very long, and the life requirement is 10 years or more. Medical safety regulations also clearly state that medical equipment must have a clear life expectancy to ensure that the equipment can provide long-term and effective service. Therefore, the power supply must have a long life. First of all, the power supply device must have a long enough supply period to avoid the discontinuation of the device and forced redesign, and the equipment medical certification must be updated; the other is the life of the power supply itself, which involves the design and device of the power supply. Factors such as the selection of the capacitor, the temperature of the capacitor, and different application conditions will have a direct impact on the life of the power supply.
5. Reliability and consistency
Power suppliers are required to have deep design experience, stable component suppliers, strict material certification systems, and complete design and production management systems to ensure that every power supply shipped from the factory meets the specifications and is stable and reliable. Of course, the power supply must have data to support its reliability, including MTBF, device voltage and current stress, device temperature rise, life, mechanical stress, hot and cold shock experiments, and even abnormal test reports, that is, deliberately setting the power supply to an abnormal state. This ensures that even if the power supply fails, there will be no smoke, fire or other situations not allowed by safety regulations.
6.Technical services
Many indicators of power supply are interrelated and directly affect the safety regulations of the entire equipment. For example, leakage current and EMC are in conflict with each other. Generally, if the leakage current is small, the EMC will be poor. However, if any one of them does not meet the standards, medical certification will not be obtained. Therefore, in the early stage of selection, be sure to closely communicate with the power supplier about power supply indicators and application key points. In addition, system EMC is often very complex, and the power supply is one of the noise sources. Therefore, power suppliers need to provide strong technical support to help quickly pass the EMC test. Finally, if the power supplier can provide relevant technical support during equipment inspection, it will be much smoother. In addition, if the medical equipment manufacturer has special requirements or standards, the power supplier must also be able to provide flexible, timely and effective technical services.
7.Quality management system
In the global market, due to human health and environmental protection requirements, all products need to comply with RoHS and REACH directives, and power supplies are no exception. In addition, due to the particularity of medical devices, the ISO organization promulgated the ISO13485 standard, which puts forward special requirements for the quality management system of medical device manufacturers, which plays a very good role in promoting the safety and effectiveness of the quality of medical devices.
8.Power price
Medical equipment is of high value, has a long service life, and performs critical tasks. Once it fails, the consequences are often serious. Therefore, medical equipment must comply with more stringent standards than consumer and other industrial equipment. Similarly, compared to the 60950 standard for general power supplies, medical power supplies need to comply with UL/EN 60601 medical standards. Therefore, the first thing medical equipment manufacturers must consider is the quality, reliability and stability of the power supply.
FAQ